Research Projects
Video-based intelligent assessment of Parkinson’s disease
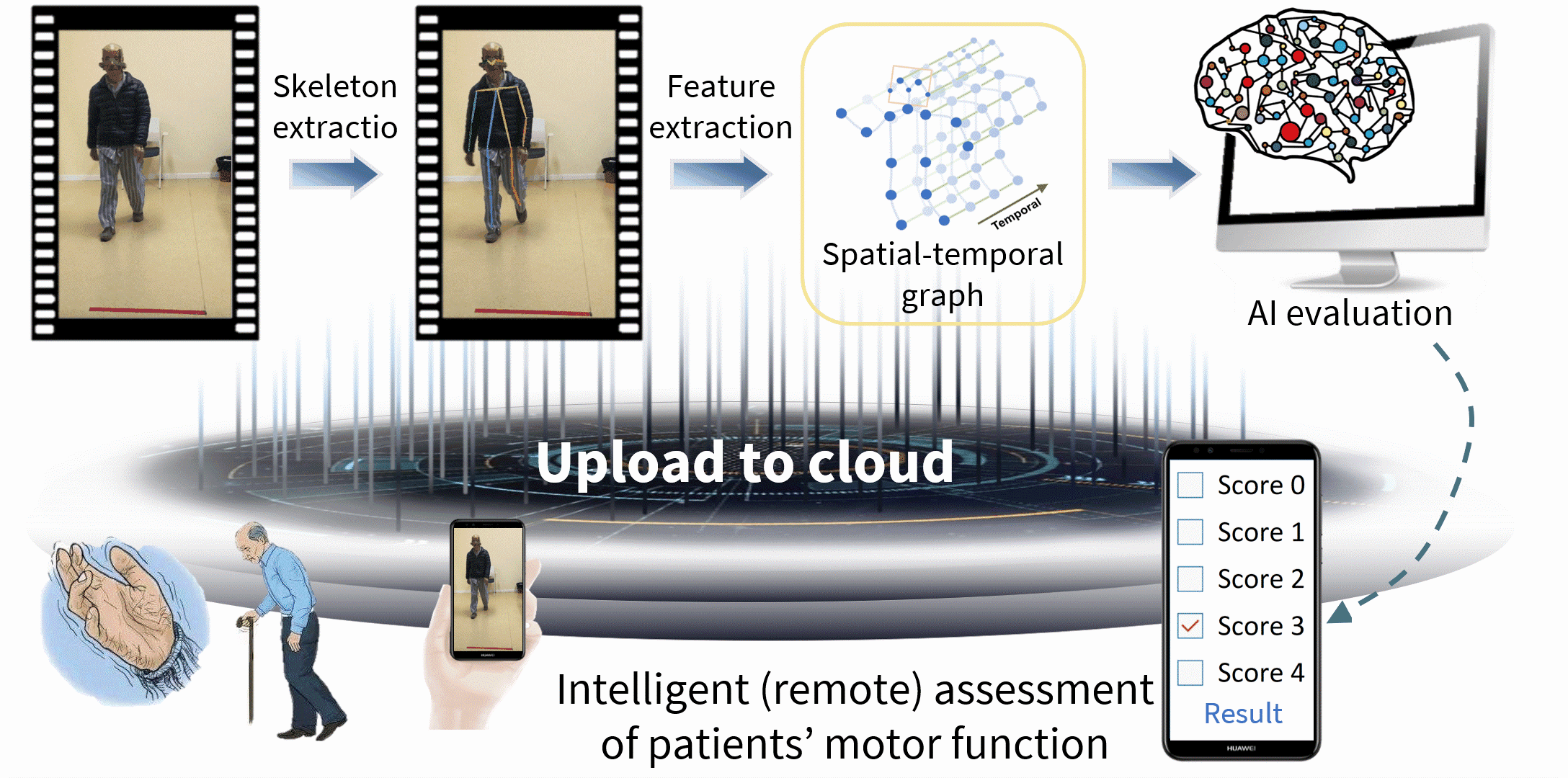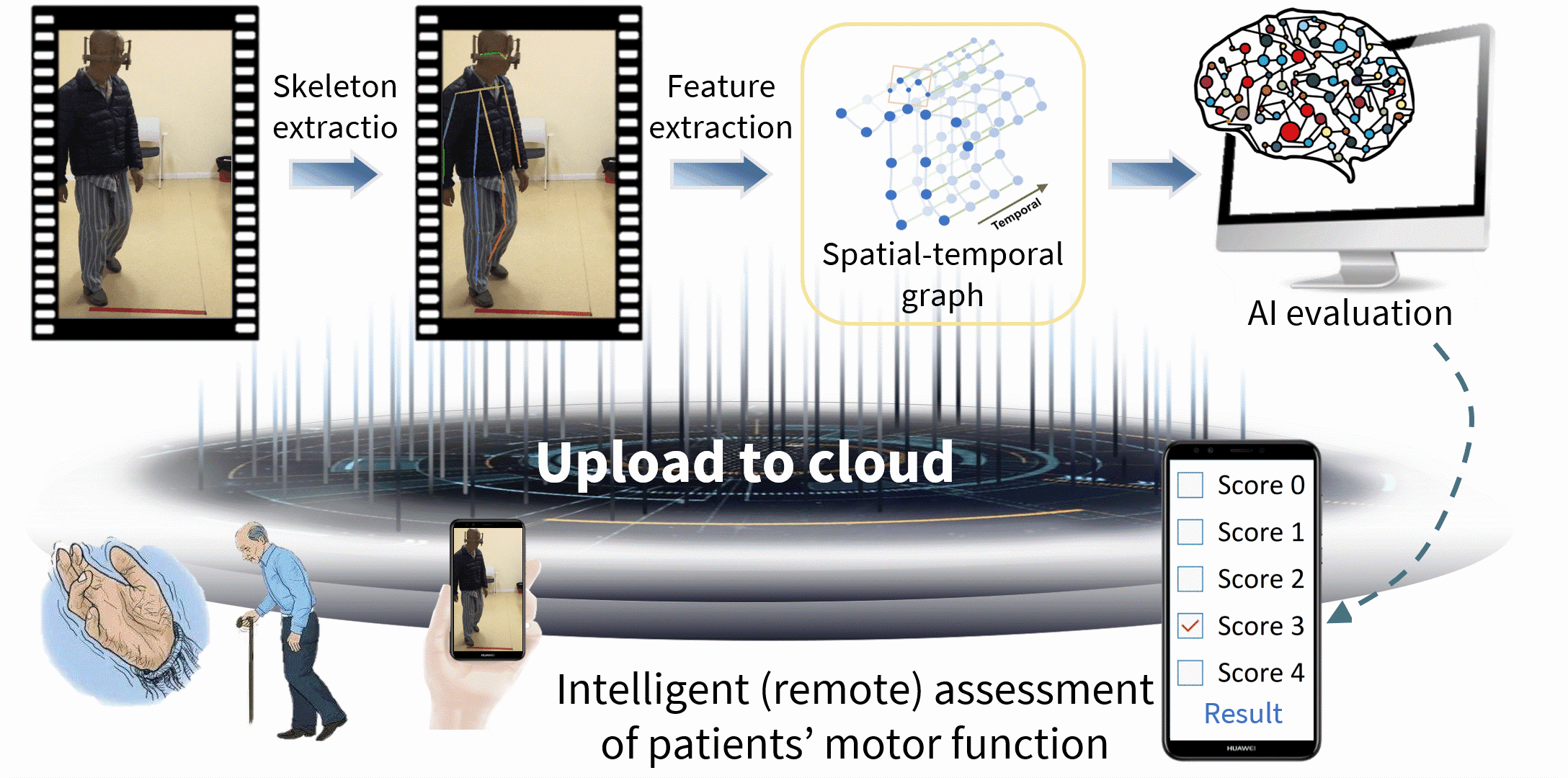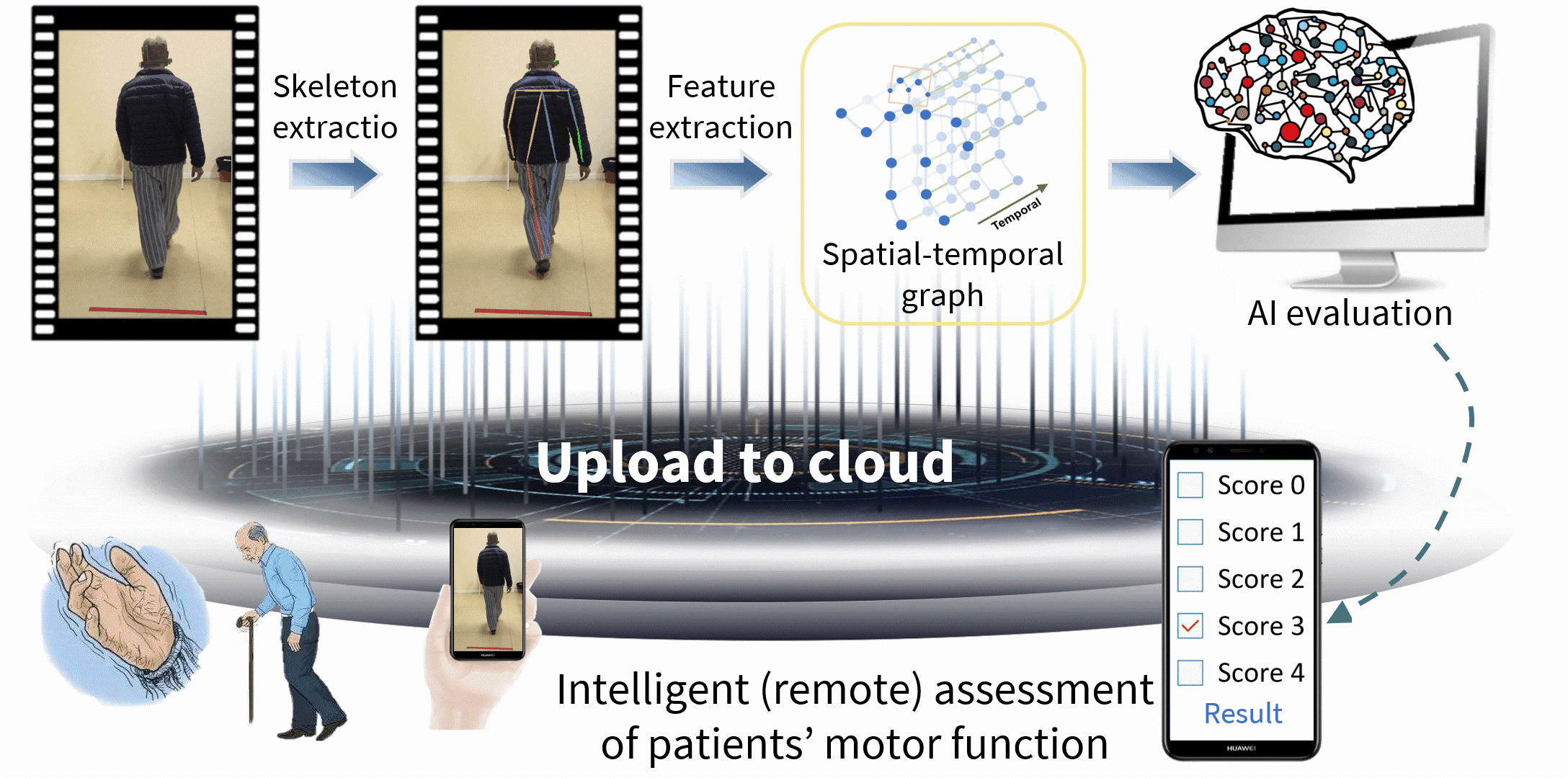
Parkinson’s disease (PD) is a serious neurodegenerative disease, and is faced with the realistic problems of few doctors, nonobjective diagnosis and discontinuous follow-ups. Currently, the Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS) is the gold standard for clinical diagnosis and evaluation of PD, and hence intelligent assessment based on this scale is very crucial. Therefore, we have systematically proposed the spatial-temporal fine-grained feature mining technology, thereby solving the key challenge in fine-grained action analysis of medical videos; on this basis, we have built the PD video-based intelligent assessment system, and realized the automated five-pointing scoring of motion tasks, including hand movements (MedIA 2022), gait (TMM 2022), toe tapping (TCSVT 2022), arising from chair (TCSVT 2022), leg agility (TNSRE 2020), and finger tapping (NEUROCOMPUTING 2021), finally achieving acceptable accuracies of more than 90%, which are higher than similar studies at home and abroad, even better than the sensor-based assessment performance. Additionally, we have expanded the above core technologies, built an intelligent assessment system for cervical dystonia based on the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), and realized automated scoring of the torticollis severity.
Medical imaging informatics system for early diagnosis and treatment of pancreatic cancer
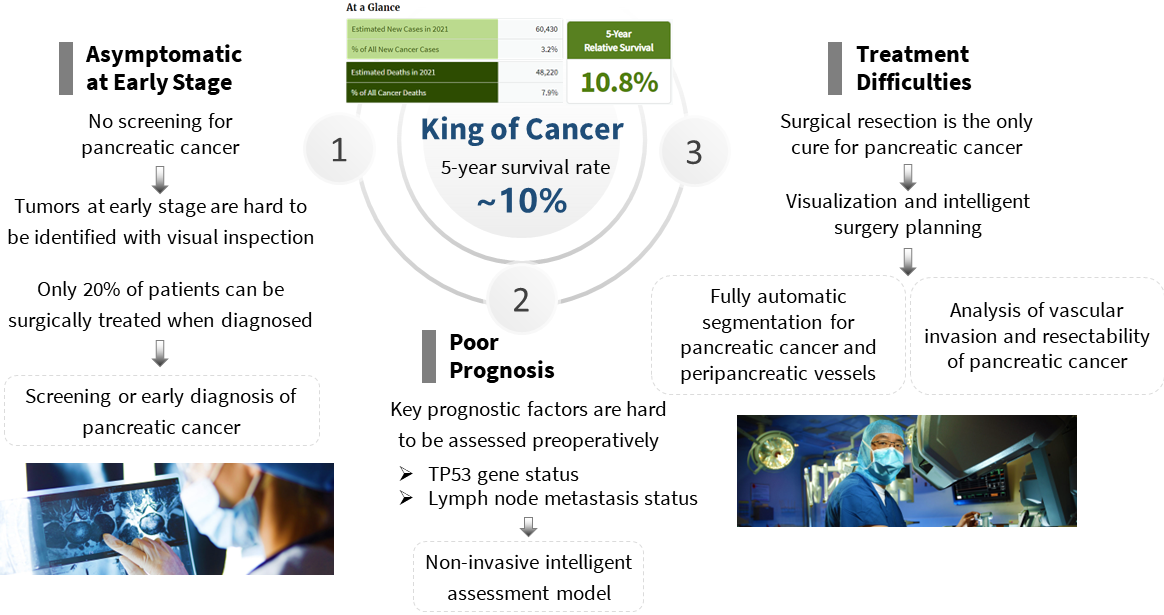
Pancreatic cancer is one of the most lethal malignant tumors and is considered as the "king of
cancers" for the following possible reasons: 1) lack of early alert indications and
effective
screening/early diagnosis instruments, resulting in most patients being diagnosed at the
advanced stage; 2) poor prognostic due to difficulty in preoperative assessment of
prognostic
risk factors; 3) surgical resection is the most effective treatment, but it is regarded as
the
most challenging general surgery due to its complicated anatomical structure and the involvement
of critical blood vessels.
To this end, we have investigated the medical imaging informatics
system for the whole process of pancreatic cancer clinical diagnosis and treatment, including:
1) early diagnosis of pancreatic cancer based on non-contrast/contrast CT;
2) genetic
markers’
status and lymph node metastasis predictionsof pancreatic cancer based on multi-modal images;
3)
segmentation of pancreatic cancer and peri-cancerous vessels, and analysis of vascular invasion
and resectability of pancreatic cancer.
Currently, our developed early diagnosis system has
been
confirmed by the multi-center data of thousands of patients; our prognostic assessment system
has also achieved accurate assessment of two key factors, TP53 gene status and lymph node
metastasis; the automated segmentation model has indicated excellent robustness and
generalizability on multi-modal, multi-phase and multi-center datasets consisting of thousands
of patients. Our ultimate goal is to empower the clinic with our medical imaging informatics
system to benefit pancreatic cancer patients.
Biomedical data mining
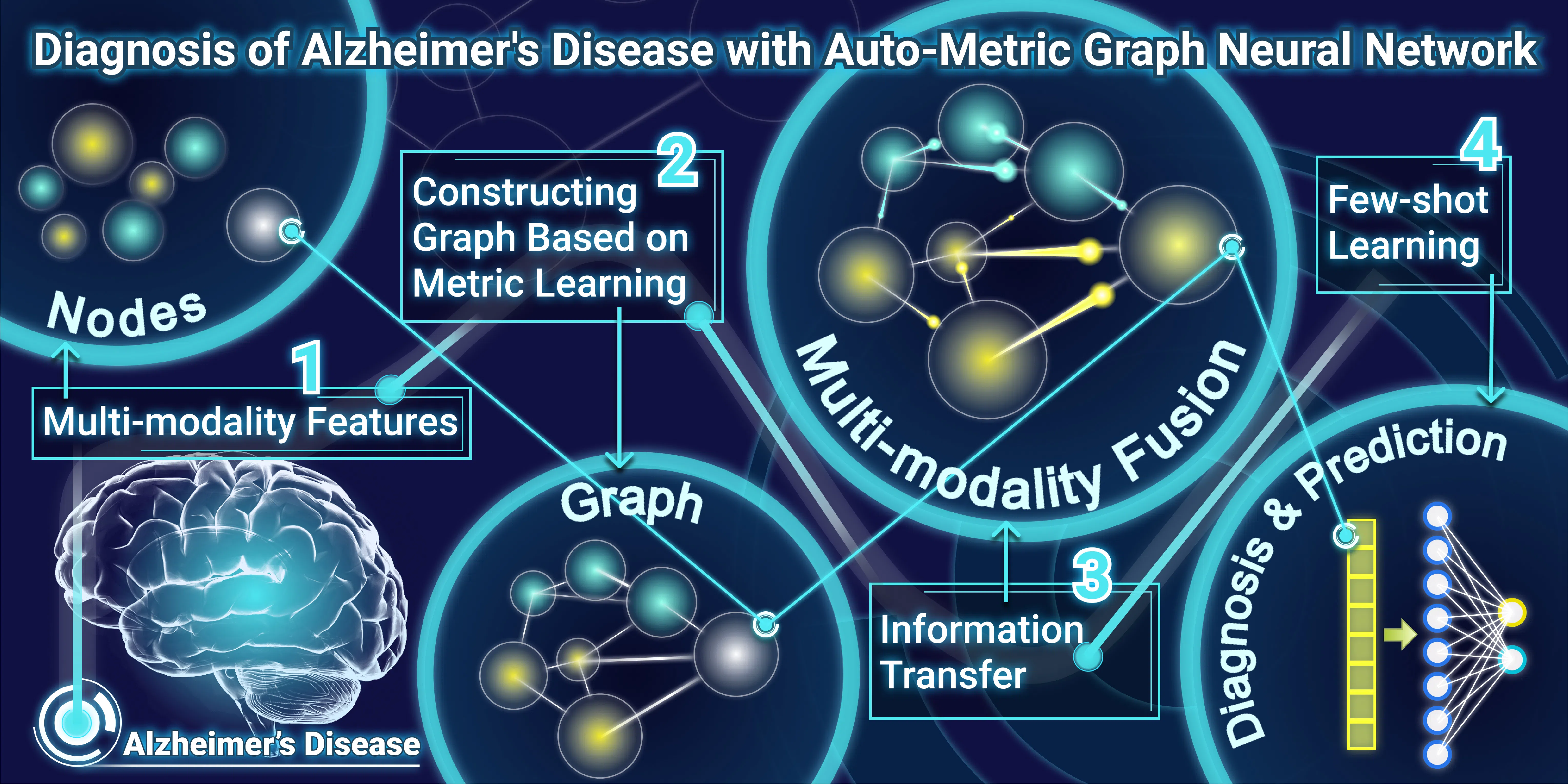
Biomedical data analysis faces technical challenges such as missing values, small sample sizes, and high dimensions. Specific clinical demands often make the collection of clinical data only partially available, resulting in missing values. This also leads to a small sample size; that is, the samples containing the same data modal are always few. Simple deletion or imputation may lose sufficient discriminative information, leading to inferior performance of subsequent classification. In addition, a certain modal of data may suffer from a high dimensionality. For example, the dimension of DNA methylation data is up to 100k, and the curse of dimensionality brings plenty of redundant information, hiding the discriminative features. We are committed to developing innovative algorithms based on graph neural networks, meta-learning, biological prior guided feature selection, and other machine learning technologies to solve the above problems.
Imaging-based intelligent analysis on true and false progression of glioblastoma
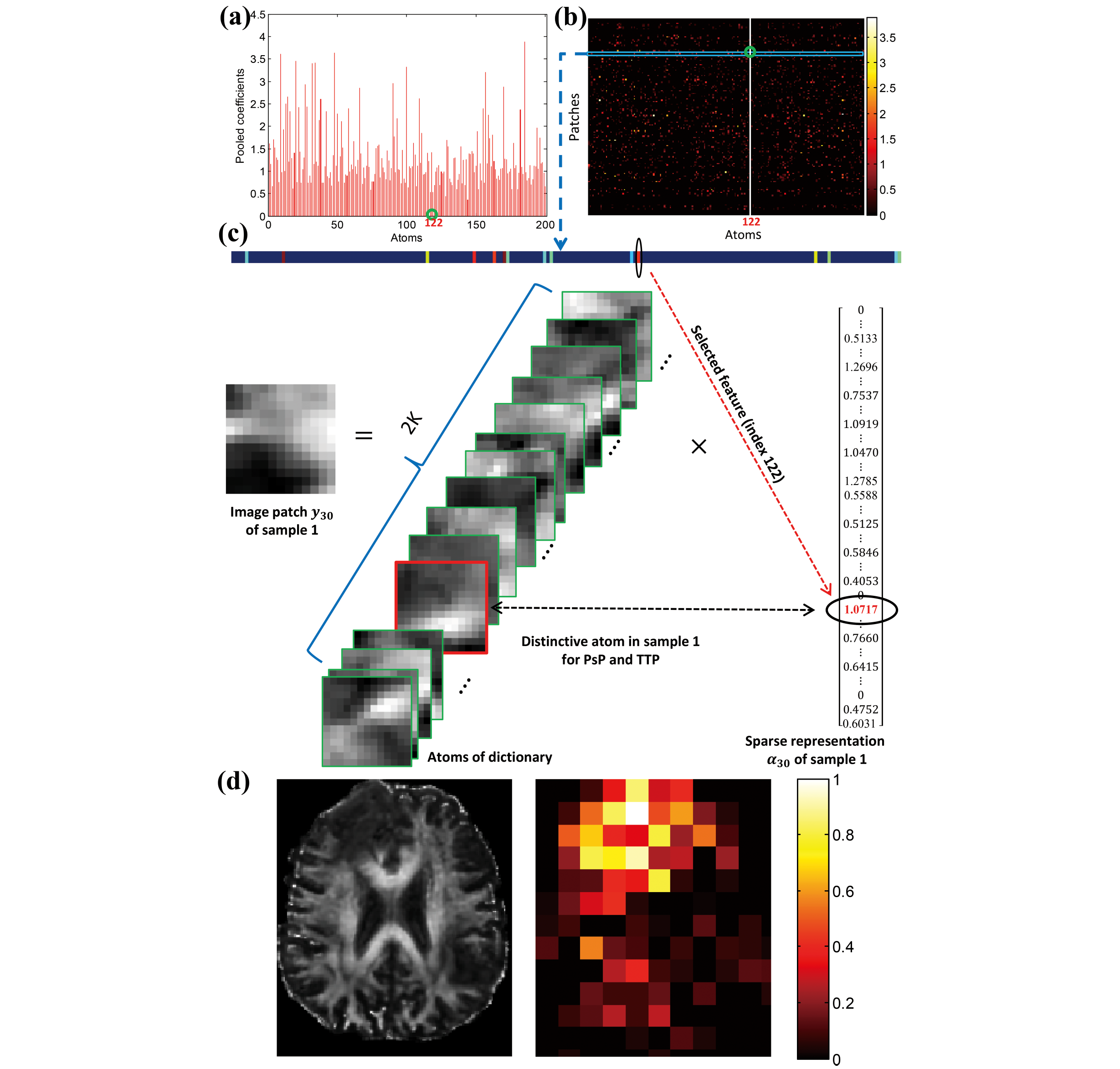
Glioblastoma (GBM) is the most lethal primary brain tumor, with a typical patient survival of 12-15 months. Approximately 40% of GBM patients who undergo surgery would develop pseudoprogression (PsP). As the imaging characteristics of PsP closely resemble those of true tumor progression (TTP), PsP cases can easily be misdiagnosed as TTP. This misdiagnosis may lead to unnecessary and potentially-harmful surgical interventions, which can further aggravate the condition of a GBM patient. Thus, to facilitate the proper treatment strategy, we have constructed a series of true-false progression diagnosis algorithms with clinical interpretability based on dictionary learning, adversarial learning, CNN and GNN. These results demonstrate that our innovative algorithms can learn discriminative fine-grained features from the small sample size, thereby affording a reliable guarantee of our current leading performance in progression diagnosis.
Highlights
-
One paper has been accepted by Nature Communications. Congratulations to Xinlu Tang and Rui Guo!
Jan · 2025 -
Xinlu Tang won the National Scholarships!
Sep · 2024 -
PC project team won the second prize of the National Innovation and Entrepreneurship Competition!
Aug · 2024 -
Rui Guo was honored as “Innovation Star” for Shanghai Jiao Tong University Postgraduates in 2023!
Mar · 2024 -
Pancreatic cancer screening project won the first prize at the 2023 Second Medical AI Innovation and Entrepreneurship Competition!
Dec · 2023